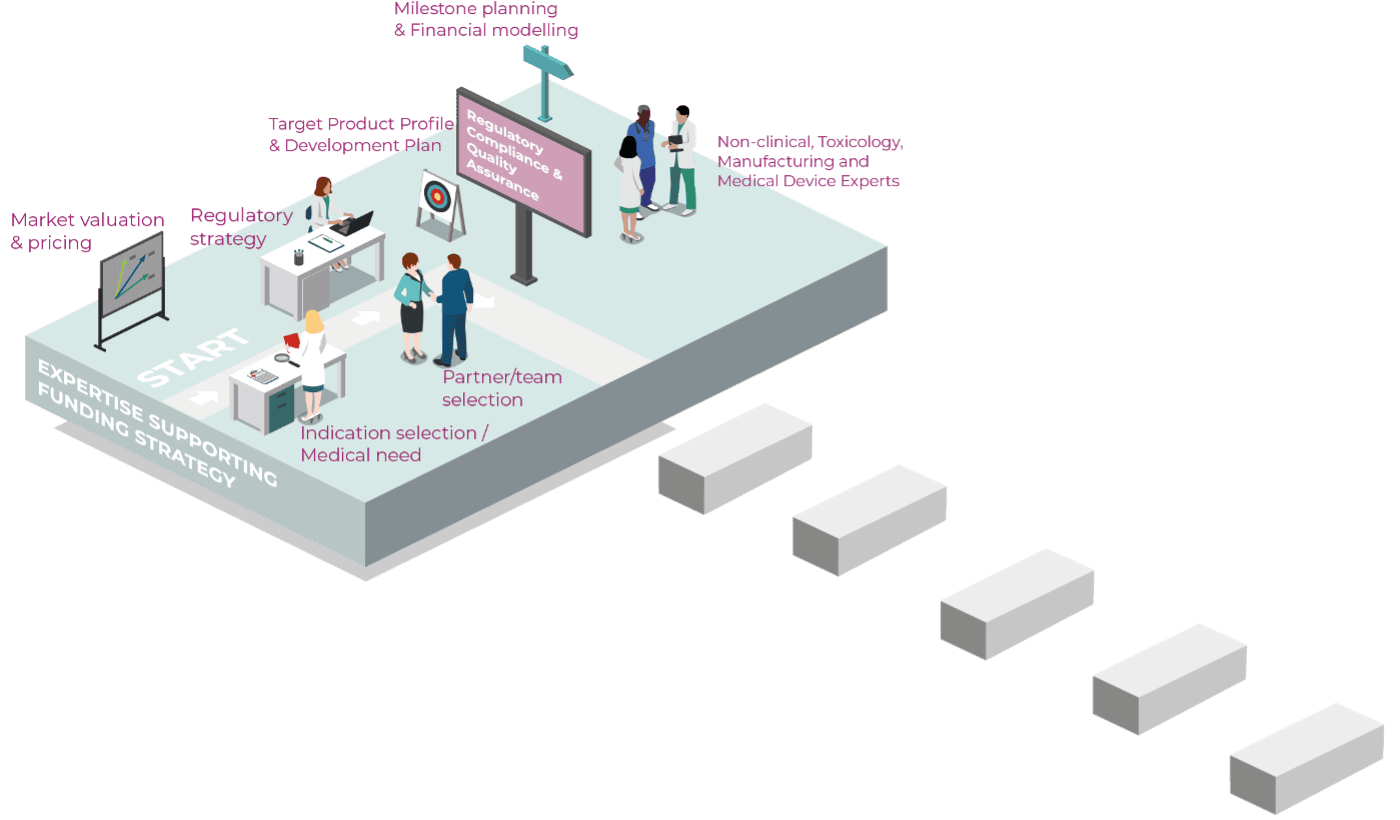
Navigating the regulatory landscape can be challenging for a small-to-medium-sized medical device or pharmaceutical company. There are many approaches to clinical development, and you want to know the most cost-effective and strategic path to success. That’s where Gouya Insights becomes your expert partner. Regardless of the project scope or development stage, we’re eager to translate your innovation vision into a roadmap that streamlines your clinical development plan and puts your product on the quickest route to market approval with the highest clinical impact.
Gouya Insights offers a full range of services to meet the demands of any clinical project, from protocol writing to project management, to vendor selection and beyond. We’ve worked with biotech start-ups as well as large pharmaceutical companies to successfully bring innovative medical and pharma products to market. Our success lies in our team’s agile and cross-functional way of working, helping break down silos and facilitating decision making, to jointly create streamlined strategies for your product.
We are more than consultants; we are your partners. We believe in your product and work passionately to make your goals a reality.
Establishment and implementation of your product´s (drug, medical device) clinical development strategy:
Our medical writers prepare concise, well-researched, GCP-compliant documents, including:
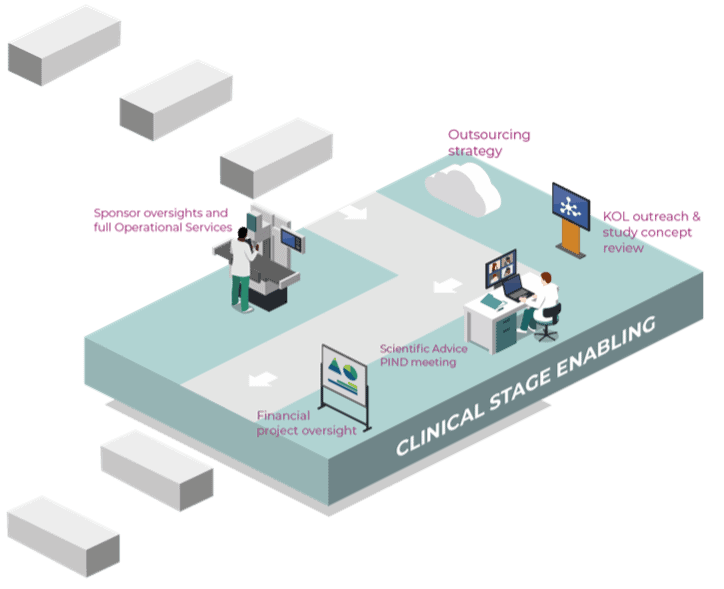
Successful implementation of the Clinical development plan. Project delivery from initiation to closure through extensive project lifecycle:
Systematic process for the assessment, control, communication and review of risks in the clinical development:
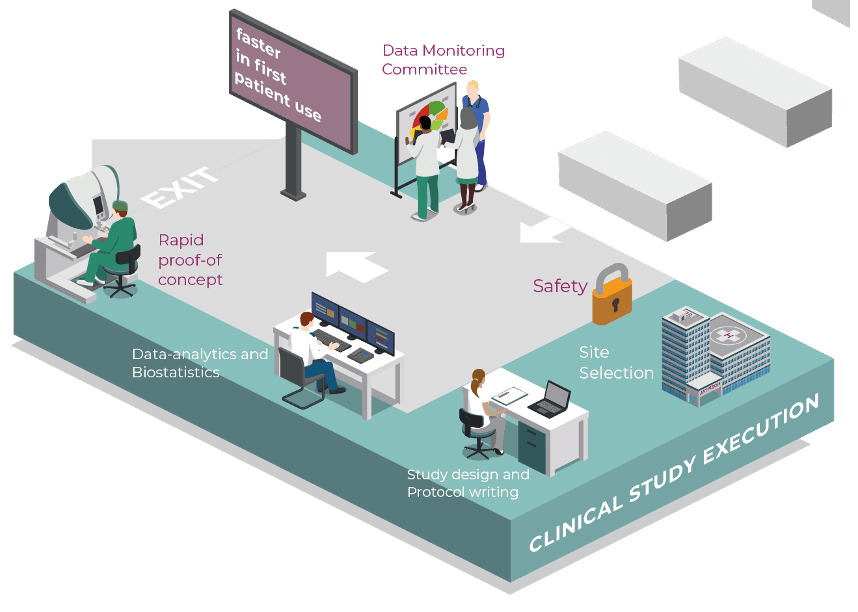
We create electronic case record designs with logic automated checks to simplify the generation of plausible data. Review and query clinical study data that impact patient safety or the overall conduct of the trial:
Use of a testing tool for drugs, devices, and techniques:
Responsibility for the conduct and reporting of clinical trials:
The pharmacovigilance and medical device vigilance team are responsible for:
Outsourcing strategy for the ideal set-up for your company:
We apply statistical concepts to design and conduct effective clinical trials. We support our clients from protocol design through regulatory submission, while our biostatistics team analysis supports the interpretation of data:
Every Monday we hold expert hours to help you navigate next steps for your project. Book now!